Facilities

GERG occupies several buildings including:
| Main Building: | 14,495 sq. ft. climate controlled |
| East Building: | 4,434 sq. ft. climate controlled |
| Portable Building: | 712 sq. ft. climate controlled |
| Ocean Sciences Laboratory: | 3,000 sq. ft. climate controlled |
| Warehouse: | 2,045 sq. ft. enclosed |
| Glider Building: | 5,000 sq. ft. enclosed |
| Machine Shop: | 2,305 sq. ft. open-bay |
GERG has ~32,000 sq. ft. of air-conditioned office and laboratory space; 3,000 sq. ft. of air-conditioned, oceanographic laboratory space; enclosed staging and storage space; and 2,000 sq. ft. of partially enclosed, roofed space for open storage, refrigerated storage, and shops. A large concrete apron connects the buildings and provides over 6,000 square feet of concrete staging and maintenance area for field equipment, including storage for trucks, laboratory and shop vans, and freezer vans. The cold storage areas include a secured “walk-in” type refrigerator and three secured “walk-in” type freezers.
GERG and the Department of Oceanography have established an equipment base for oceanic and coastal marine research including sample collection and analyses capabilities. GERG preforms oceanographic, geochemical and environmental analyses. GERG's infrastructure of analytical instrumentation, laboratory facilities, and computers provides the tools needed to satisfy the requirements of these analyses. GERG offers complete sample preparation laboratories as well as instrumentation for the analysis of salinity, dissolved oxygen, nutrients, trace elements, aliphatic hydrocarbons, aromatic hydrocarbons, petroleum biomarkers, total scanning fluorescence, PAH metabolites, pesticides, polychlorinated biphenyls (PCBs), planar PCB, PBDEs, dioxin/furans, semi-volatile organic compounds, volatile organic compounds, and other organic compounds. GERG has just been designated as part of the Texas A&M Mass Spectrometry Core Subunit for Applied Mass Spectrometry (AMS) with Dr. Anthony Knap as the Director of the AMS. GERG will provide instruments to those needing to analyze various analytes in environmental samples and other Matrices.
The Sample Control Laboratory is isolated from the main laboratories and is a controlled access area. This facility and the procedures used provide for the receipt, safe handling, and secure processing of environmental samples. The sample receipt area is isolated from the main laboratories and includes approximately 500 square feet of space. This area is lined with benches with chemically resistant bench tops and isolated room ventilation providing adequate space to safely process samples in a contaminant-free environment. A sample receipt area provides for the safe, controlled receipt and processing of samples under chain-of-custody SOPs. This area is partitioned from the sample preparation area where sample homogenization and aliquoting are performed. GERG has a Class II Type B2 biological safety cabinet which allows for preparation of potentially contagious samples including human tissues. The initial processing of environmental samples is further supported through the use of two freeze driers, three drying ovens, two meat grinders and a band saw. The cold storage, controlled access areas occupy more than 1,400 square feet of space. These “walk-in” freezers and refrigerators are secured and the temperatures are monitored to detect system failures. Active and archived samples are stored in these areas under chain-of-custody SOPs. Samples may be stored in this area for periods of 90 days or greater. Sample extracts and standards are stored in secured, dedicated refrigerators and freezers apart from the sample storage area.
GERG's main laboratories occupy more than 6,950 square feet of space. This provides more than 250 square feet of laboratory space per research staff. Access to all areas within GERG is controlled to ensure confidentiality of samples and data. A variety of digestion, extraction and purification techniques are used to meet a project's specific objectives. GERG malor equipment is listed in Table 1. GERG has two Dionex Accelerated Solvent Extractors (ASE). When samples for organics are isolated and purified, sample extracts are analyzed for specific analytes using high resolution fused silica capillary gas chromatography (HRGC). A multitude of gas chromatographic detectors are available including flame ionization, electron capture, mass spectrometers and a high resolution mass spectrometer. Analytical instrumentation is computerized and automated for rapid and efficient sample throughput. GERG’s organic analyses are provided by 6 gas chromatograph with mass spectrometers (GC-MS) detectors, 1 dual-channel gas chromatographs with two electron capture (GC-ECD) detectors, 2 gas chromatographs with micro-electron capture (GC-uECD) detector, 4 gas chromatographs with flame ionization detectors (GC-FID). GERG has a GC with a high resolution mass spectrometers (GC-HRMS) for the analysis of dioxin/furan and dioxin-like PCBs. For other trace organic analyses, GERG has 2 high performance liquid chromatographs (HPLC), 1 HPLC coupled to a mass spectrometer (HPLC-MS). All instruments are fully automated with injectors and computers for data acquisition.
Most recent acquisitions in analytical equipment in 2017 is a Waters/Agilent 6470 triple quadrupole LC-MS/MS, an Agilent Ion Mobility Q-TOF LC/MS, an Agilent 7010 GC/MS/MS Triple Quadrupole EI system and a Leco GC/GC 2 dimensional system with a Time of Flight Mass spectrometer. The IMS and LCQQQ are housed in a Biological Safety Laboratory 2 for the analysis of human cells as well as environmental samples.
GERG has a six channel Astoria analyzer for nutrient analyses and 3 automated oxygen titrators. GERG can perform salinity analyses on either a Guildline Autosal 8400B or 8400A.
GERG’s trace metal laboratory has 2 forced air hoods and 1 perchloric acid hood housed in an isolated controlled access area where samples are digested. Teflon vessels and sufficient lab-ware is available for use in acid digestion of samples. The inorganic sample preparation room is adjacent to the inorganic instrument room and both rooms have a heating and ventilation system isolated from the remainder of the GERG laboratory. The laboratory has a PerkinElmer FAMI 400 cold vapor mercury atomic absorption spectroscopy (CVAAS) system with an AS 90 Auto Sampler. Other trace metals are determined by inductively coupled plasma mass spectrometry (ICP/MS) using a PerkinElmer NexION ICP-MS located at the Texas A&M University Chemistry Trace Characterization facility. GERG also can perform metal analyses by neutron activation at Texas A&M University research reactor.
Approximately 2,500 square feet of office space exists for the compiling, packaging, and shipping of analytical data and other deliverables.
Ocean Observing
For the more than 40 years, the GERG leadership and Ocean Sciences Group has been heavily involved in oceanographic and ocean observing systems design and operations, and global observing system vision efforts. Major programs have been pursued in the Gulf of Mexico, Caribbean Sea, Sea of Oman and Arabian Sea, coastal Africa and Antarctica, and the Atlantic and Southern Oceans. The hallmarks of these projects and systems designed, deployed, and operated are sustained real-time observations that address issues of societal relevance such as climate change, oil spill response and mitigation, harmful algal blooms, coastal hypoxia, and ocean acidification.
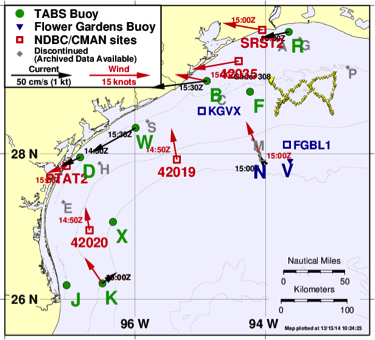
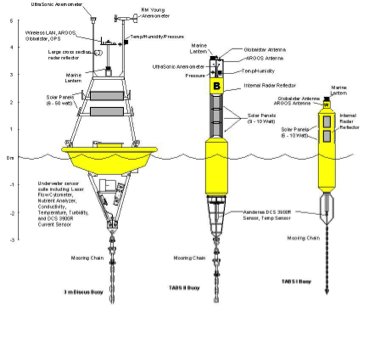
Figure 1. TABS buoy system locations (http://tabs.gerg.tamu.edu). Left: TABS buoy designs.
TABS. Since 1995, GERG has designed, deployed, operated, and maintained the Texas Automated Buoy System (TABS). TABS is the signature ocean observing system in the Gulf of Mexico and among the earliest and longest continuously operating coastal ocean systems in the world. This unique system of buoys consists of an array of advanced integrated sensors that telemeter oceanographic and atmospheric conditions in near-real time in support of state and national agency oil spill monitoring and trajectory modeling.
These systems serve the wider scientific goals of the U.S. Integrated Ocean Observing System (IOOS) by making data available to the general public and other coastal ocean-observing associations such as the Gulf of Mexico Coastal Ocean Observing System Regional Association (GCOOS-RA: http://gcoos.org). Generally, the scientists and technicians of GERG are passionate about observing systems operations and technology and participate in international community workshops, meetings, and conferences to share their expertise and participate in the efforts to improve and advance ocean observing technology and applications.
The buoy operations have a dedicated facility of more than 5000 sq. ft. of air-conditioned space for buoy maintenance, construction, construction, and repair. The facility includes space for electronic, computer, and sensor maintenance and calibration. Additional there is more than 10 sq. ft. of concreted apron space for buoy storage and servicing and a dedicated machine and welding shop.
Ocean Gliders and Autonomous Ocean Vehicles. The TAMU College of Geosciences, Department of Oceanography and GERG have made significant investment in the new and emerging technology of ocean gliders and autonomous ocean vehicles. Currently, TAMU owns and operates six of these vehicles. Four of these vehicles are the Teledyne Webb Research (TWR) Slocum G2 Ocean Gliders (S/N 307, 308, 540, and 541). The gliders are rated for operations in depths of up to 200 m; one glider (S/N 199) rated to 1000 m is owned by TAMU-Galveston and is operated by GERG. All gliders are currently equipped with SeaBird CTD (designed for glider application), ECO-PUC fluorometer, and RINKO fast response dissolved oxygen sensor. Several GERG/TAMU electronic technicians are trained in the operation and maintenance of the Gliders; GERG/TAMU has four glider technicians trained to pilot the gliders on missions. GERG also has several buoyancy pumps to efficiently task G-2 gliders with shallow (fresh) or deep (salty) missions.
GERG also two autonomous surface vehicles: the Autonaut and the Wave Glider. The Autonaut, manufactured by MOST (MOST, Inc.; http://www.autonautusv.com)) of the UK, harvests energy from waves for propulsion, but it makes use of a combination of solar energy and methanol fuel cells for system power. Autonaut is equipped with conductivity, temperature, and ADCP for measuring underway current profiles, a Wetlabs ECO FLNTU for measuring turbidity and chlorophyll. In March 2015, TAMU/GERG acquired one Liquid Robotics (http://www.liquidr.com) surface SV3 Wave Glider which is equipped with a flow through system for measuring waves, pH, CO2, water temperature, conductivity, CDOM, chlorophyll and turbidity. Like Autonaut, the Wave Glider uses surface gravity waves for propulsion. Both ASVs also collect wind speed and direction, air temperature and barometric pressure.
In April 2014, GERG opened the permanent glider facility, known as the Glider Center for Autonomous Vehicle Engineering. This air-conditioned 1250 square-foot dedicated glider facility is for servicing, preparing and testing autonomous underwater vehicles. Located at GERG in College Station, the facility is maintained by two full time glider pilot/technicians who in conjunction with the scientific authority map out, plan and simulate missions. Additionally, six others have received Slocum glider training and are used as temporary relief for the glider pilots. The lab is equipped with a gantry system to help move gliders in and out of a 950 gallon salt water test tank used for ballasting and testing the underwater gliders.

In June 2015, GERG expanded the facility to include an additional 700 square feet to accommodate the two new autonomous surface vehicles. Part of the expansion includes installation of a larger 2244 gallon saltwater tank to accommodate new larger vehicles.
The Glider facility contains two glider workbenches, powered lift, large screen computer monitors, Internet connection, conferencing technology, desk area, communication facilities (for short wave communication), and ballasting tank. The gliders are capable of operations using alkaline and lithium batteries. Deployment durations are dependent on many factors and must be determined during the mission-planning phase of operation. Mission planning is done in concert with the chief scientist, who dictates the scientific objectives of the mission, and the glider pilot, who dictates system capabilities, and formulates glider systems power and communications requirements. The scientist is required to secure insurance for the gliders against loss or damage for the duration of the mission (including transit to and from the water).
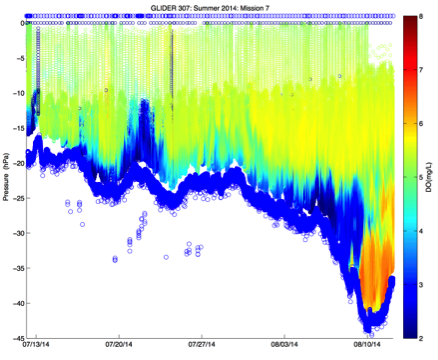
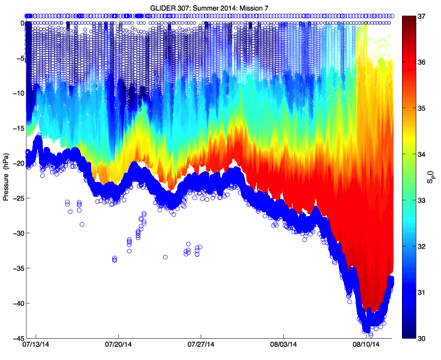
Figure 2. Time-series versus depth for two glider missions on Louisiana Shelf during summer 2014. Left: dissolved oxygen concentration (mg/L) during July-August south of Atchafalaya Bay region. Right: practical salinity (from conductivity) during July-August. Figure 2 shows individual observations; no contouring or smoothing was done. The missions were specifically designed to obtain observations of near bottom oxygen concentration in the Deadzone region of the northern Gulf of Mexico.
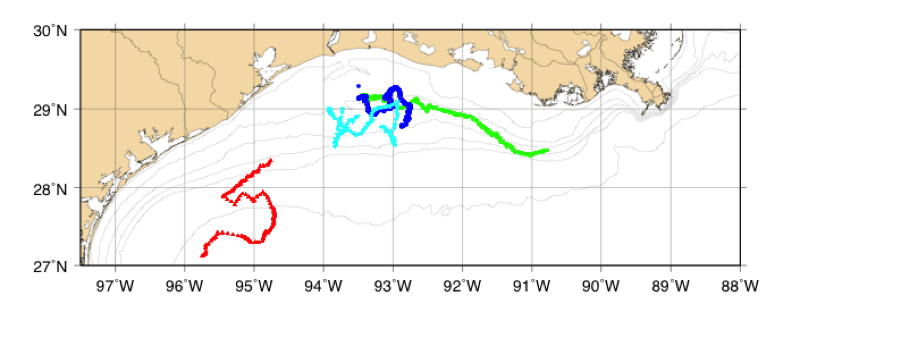
Figure 3. Planview of northern Gulf of Mexico, Texas-Louisiana Shelf. Colored symbols depict glider locations during four deployments between October 2013 and September 2014. Red triangle: October 2013, dark blue circle: July 2014, green square: July 2014, cyan inverted triangle: August 2014. Isobaths shown are 10, 20, 30, 40, 50, and 500 m.
Texas SeaSondes (Coastal Radar)
TAMU is improving the observing capability along the northern Gulf of Mexico by implementing coastal ocean SeaSonde stations from the US-Mexico border to the Texas state border with Louisiana. The SeaSondes will provide real-time and continuous surface current mapping and wave monitoring with ranges of up to 200 km from the coast. The observations will supplement the in situ observations of the TABS network and produce accurate two-dimensional maps with 0.5 to 3 km resolution within the system footprint. The SeaSonde network is scheduled for implementation in July 2015 and fully operational in August 2015. SeaSonders are manufactured by CODAR Systems (US).
Ferry box Applications
Ships of opportunity are plentiful in the world ocean. TAMU has implemented a sizable and dedicated effort to the design, construction, and deployment of autonomous ship-based systems to be placed on voluntary ships. The systems, dubbed ferry-boxes, are already in continuous service at a few locations around the world. The ferry-boxes offer the opportunity for sustained and near continuous real-time observations in ocean regions that are heavily trafficked. Typical ferry-boxes use a ship flow-through system and provide observations of temperature, salinity, and fluorescence along ship tracks. Advanced systems can be configured to provide water quality, biological information, current velocity, meteorological parameters, and chemical contaminants. All ferry-box systems telemeter data to a shore-based receiving station where data are processes and disseminated to interested parties. Ferry-box systems are designed for automation with little to no servicing training from ship’s crew. Ferry boxes can be deployed on cruise lines ships, ferries, work boats, commercial and recreational fishing vessels.
